Thyroid Function and the Natural History of Depression: Findings From the Caerphilly Prospective Study (CaPS) and a Meta-analysis
Abstract and Introduction
Abstract
Objective: Low thyroid function has been associated with depression in clinical populations. We have examined whether thyroid function in the normal range is associated with
minor psychiatric morbidity.
Design: Prospective cohort study of 2269 middle aged men (45–59 years) with thyroid function (total T4 only, TSH unavailable) measured between 1979 and 1983 and with repeat
measures of minor psychiatric morbidity (GHQ-30) over a mean of 12·3 years follow-up. We also undertook a systematic review and meta-analysis of population-based studies examining
thyroid function and mood.
Results: There was a positive association between total T4 and chronic psychiatric morbidity (odds ratio 1·21, 95% CI 1·02–1·43, P= 0·03), but
this was consistent with chance after adjusting for social class, alcohol and smoking behaviours. The association with incident and recovery from psychiatric morbidity was weaker and consistent
with chance.
We identified seven eligible studies, from our systematic review and included six studies, including our own, in a meta-analysis. The pooled estimate showed a positive association (odds ratio
1·12, 95% CI 1·02–1·22, P-value = 0·01) between depression and T4 and an inverse association with TSH (odds ratio 0·92, 95% CI
0·88–0·97, P= 0·0007) with no evidence of heterogeneity or publication bias.
Conclusion: The results from CaPS and our meta-analysis are consistent and suggest that, if anything, higher levels of thyroxine in the normal range are associated with increased risk of
depression. The effects of thyroid hormone on mood may differ in normal populations and patients with clinical thyroid dysfunction.
Introduction
The conventional wisdom is that clinical hypothyroidism may be a reversible cause of depression. Medical guidelines for clinical practice, produced by the American Association of Clinical Endocrinologists, state that 'the diagnosis of subclinical or clinical hypothyroidism must be considered in every patient with depression.'[1] In the case of subclinical hypothyroidism (defined as normal T4 with raised TSH), a narrative review[2] rated differences in prevalence, severity of mood symptoms and quality of life as not providing conclusive evidence of a link.
There are biological reasons why low thyroid function could cause depression. Brain 5-HT activity may be reduced by hypothyroidism[3] or thyrotropin-releasing hormone (TRH) may itself be a neurotransmitter with antidepressant properties.[4] However, the existing literature consists mostly of case–control and small cross-sectional studies, which are difficult to interpret. The selection of an appropriate control group in case–control studies is often difficult and may be biased and it is possible that thyroid function is itself altered by depressed mood amongst cases ('reverse causality').
A large cross-sectional study looked at risk factors for depression and anxiety in 30 600 subjects (without a prior history of thyroid dysfunction). The authors reported that neither thyroid function[5] nor the presence of anti-TPO antibodies[6] was associated with either depression or anxiety. Even overt hypothyroidism was not associated with mood disorders though patients already on thyroxine therapy were excluded. This study noted that the prevalence of depression was equal for both men and women, which is inconsistent with the general psychiatric literature[7] though this may reflect cultural or methodological variations.[8] Only one previous cohort study has examined this topic. It found no association between free T4 or TSH and a continuous measure of depressive symptoms[9] over a relatively short follow-up period (3·7 years). However, the population was elderly with a very restricted age range (85–89 years), which may have masked an association because of a healthy survivor effect as well as limiting its generalizability. The prevalence of depression also reduces with age after retirement.[10]
The aim of this study was to examine whether thyroid function within the normal population range was associated with the natural history of minor psychiatric morbidity (depression and anxiety). We have examined whether thyroid function was associated with the risk of incident, chronic and recovery from minor psychiatric morbidity in an unselected population of middle-aged men.
Materials and Methods
Population
The Caerphilly Prospective Study (CaPS) was initially set up as a prospective study of cardiovascular disease and related outcomes in men aged 45–59 years, although it has since broadened to include a wide range of outcome measures and phenotypes. Briefly, all eligible males living in Caerphilly, South Wales, UK, and adjacent villages were identified from electoral rolls and general (family) practice registers. Recruitment (phase I) took place between 1979 and 1983 and 2512 men entered into the study from a sample of 2818 men (89%).[11] Ethical approval was given by the Ethics Committee of the Division of Medicine of the former South Glamorgan Area Health Authority.
Smoking status was obtained from a self-completion questionnaire and a detailed alcohol history was obtained as part of a food frequency dietary questionnaire. The equivalent volume of pure alcohol in millilitres per day was then calculated and then converted into units per day. Socioeconomic position was classified according to the Office of National Statistics' occupational classification (this is an ordinal scale with higher values indicating lower socioeconomic status). All men who were still known to be alive and residing in the area were invited to complete questionnaires and re-attend a clinic on three further occasions (phases II, III and IV) between 1984 and 1997·[12] At each phase, a full drug history was obtained, which allowed the identification of antidepressant medications and thyroxine therapy.
Thyroid Function
All subjects had an early morning blood sample taken at phase I, which reached the Department of Biochemistry at the University of Wales College of Medicine within five hours of venesection. For those subjects unable to have a morning sample taken (for instance, owing to shift work) blood was taken at a convenient time. T4 levels were assayed using an in house radio-immunoassay. In a random subset (n= 120), T3 resin uptake was also measured (using Thiopack3 [Amersham]), allowing the free thyroxine index (FTI) to be calculated as the product of total T4 and T3 resin uptake. To examine the reliability of the measures, a subgroup of men (n= 128) had split samples, which enabled us to calculate the coefficient of variation.
Assessment of Mood
The 30-item General Household Questionnaire (GHQ-30)[13] was given to participants to self-complete at phases II, III and IV. For each symptom or item of behaviour, respondents must say how often this has been experienced over the past few weeks compared with usual on a four point response scale (e.g. more so than usual, same as usual, less so than usual, much less than usual) though the wording of the scale is different depending on the question. The subjects' responses can be used to derive a total score. This was validated at phase II amongst a stratified sample of 97 subjects who had domiciliary interviews carried out by a psychiatrist trained in the Clinical Interview Schedule (CIS)[14] and blinded to the GHQ scores.
There are several methods for scoring GHQ-30 responses.[13] The conventional GHQ scoring system allocates 0, 0, 1 and 1, respectively, to the four response categories. This gives a maximum score of 30 and cases are defined as scoring five or above. One criticism of this approach is that it may fail to detect long-standing mood disorder as subjects with chronic symptoms may respond with the 'same as usual' or 'no more than usual' options. Goodchild and Duncan-Jones proposed a 'chronicity' scoring system,[15] which subdivides questions into 'negative' and 'positive' items and allocates 0 or 1 for the second response ('same/as much as usual') accordingly. Thus, 1 is given for subjects who say they experience a negative symptom or item of behaviour as much as usual, and 0 if a positive item is reported in this way. Again there is a maximum score of 30, but here the case threshold is defined as 13 and above. Finally, a simple Likert scoring method has been proposed to examine population-based distributions with less emphasis on dichotomizing subjects into clinical disease or normal. It allocates 0–1–2–3 to the four response categories. Here the maximum score is 90 and a suggested case threshold is 40 and over.[13]
Anxiety was measured using the 20 item trait scale of the State Trait Anxiety Inventory (STAI) resulting in a total score of between 20 and 80 (higher score indicating greater general anxiety).[16]
We chose, a priori, to define three case groups and examine the natural history of minor psychiatric morbidity across the life course;[17] (i) 'Incident' morbidity was defined as a subject whose GHQ score did not reach the threshold for caseness at phase II but did at either phase III or IV. (ii) 'Chronic' morbidity was defined as a subject whose GHQ score reached caseness at either phases II, III and IV or III and IV. (iii) 'Recovered' morbidity was defined as a subject with minor psychiatric caseness in phase III only. We did not include subjects who were cases at II and then recovered in this group as we could not be sure that they had not been chronically depressed or anxious prior to baseline. Recovered cases are therefore a subgroup of the incident cases. If the GHQ score was normal but the subject was on an antidepressant medication, then we used the drug data to over-ride the GHQ score, i.e. we assumed that they were clinically depressed but had made a good pharmacological response.
Statistical Analysis
We calculated the coefficient of variation of the thyroid function tests by dividing the standard deviation of the within-subject difference by the mean of the split samples and multiplying by 100. The association between T4 and minor psychiatric morbidity was examined using T4 continuously and also by quartiles of T4. The time of sampling was ignored as we found there was no association between this and T4 levels.
Z-scores were computed for T4 to present effects for a change of one standard deviation. We also examined the model using a dummy variable based on quartiles to examine for nonlinearity. We chose to use the normal GHQ weighting for the analysis of the incidence morbidity and the chronicity weighting for the chronic and recovered morbidity. We undertook a series of multivariable logistic regression models (odds ratios, 95% confidence intervals and P-values) to quantify the association between thyroid function and risk of morbidity. Model 1 simply adjusted for age. Subsequent models (models 2–4) then adjusted for socioeconomic position, alcohol consumption, and smoking status as these variables could act as potential confounders or intermediaries. Finally, model 1 was repeated with the STAI trait score included as both a continuous and a categorical variable (the latter divided into the top quintile; 'high anxiety', and the remainder). We did this as the GHQ caseness captures both depressed mood and anxiety. Adjustment for STAI should therefore diminish any association of thyroid function and morbidity if this was due to anxiety-related symptoms but not effect the association if it is due to depressed mood.
Systematic Review of the Literature
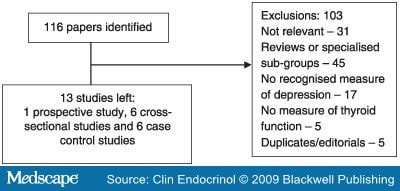
A search of MEDLINE (1966–2007), EMBASE (1980–2007) and PSYCINFO (1970–2007) was undertaken in May 2007. The search strategy and study selection methods are reproduced in Fig. 1. Our inclusion criteria were (a) adult human studies with both (b) a measure of thyroid function as exposure and (c) a recognized measure of depression as an outcome measure. Exclusion criteria were (a) duplicate publications and (b) editorials/letters. Meta-analysis was performed in accordance with the guidelines of the Meta-analysis of Observational Studies in Epidemiology Group.[18]
Figure 1.
The '$' symbol is a wild-card used when searching databases, allowing a more thorough search to be conducted. For instance, depress$ will search for depressed, depression and depressive.
Because each study analysed and/or presented the associations between thyroid function and mood in different ways, we attempted to contact all the lead authors of the studies that were included. In each case we asked them to either re-analyse their data using a standardized approach or provide us with an anonymized data set. Z-scores were computed for the thyroid measure used (which was log-transformed where this had been done in the original publication). A random effects model was used to pool odds ratios. The linearity of the data sets was tested, first by examining the effect estimates across quintiles and then more formally using the 'fracpoly' command in Stata, which tests a large number of polynomial models.
Results
A total of 2269 (90·3%) men had total T4 measured at phase I. The mean value for T4 was 92·5 nmol/l (SD 18·3 nmol/l), which was consistent with the laboratory normal range of between 55 and 150 nmol/l. 31 (1·4%) and 13 (0·6%) men were hypo- and hyperthyroid, respectively, based on this reference range. One man was taking thyroxine at baseline and was kept in the analysis. The coefficient of variation for T4 was 10·7% from split samples. This is comparable to data from contemporary studies.[20]
The mean follow-up period was 12·3 years (SD 2·9 years). Over this time the number of subjects available for assessment of depression fell from 2201 to 1493. There was no difference in the T4 of subjects who completed all four phases of follow-up, compared with those lost to follow-up (mean T4 nmol/l: 92·7, n= 1118 vs. 92·2, n= 1151, P-value for difference 0·54) Subjects lost to follow-up had slightly higher GHQ scores (chronicity weighted) at baseline than those who were continued to be followed up (mean 5·52 compared with 4·98 for subjects still present in phase IV; P-value for difference 0·03).
We identified 246, 188 and 76 men with incident, chronic and recovered minor psychiatric morbidity, respectively. 70 subjects were on an antidepressant medication (including one man on lithium who was excluded from then analysis owing to the effects of lithium on the thyroid gland) at any phase and 28 subjects had their caseness adjusted because of their medication use (see Methods). Phase II GHQ-validation found sensitivity and specificity of 69% and 71%, respectively, for GHQ caseness compared with the total weighted score from the CIS.
Table 1 provides summary data for the key variables. The majority of our subjects were manual workers who were regular drinkers and smokers. Table 2 shows the association between total T4 and incident minor psychiatric morbidity. The odds ratio was 1·08 (95% CI 0·93–1·27; P= 0·32) for a 1 standard deviation change in T4 levels. This was hardly altered after adjustment for other covariates (model 4 1·10, 95% CI 0·93–1·30; P= 0·25). Lower socioeconomic status was associated with increased risk of morbidity even after adjustment for smoking and alcohol consumption. We checked for non-linearity by repeating the analyses using a dummy variable derived from quartiles. Although the data showed a weak J-shape relationship, formal testing for departures from linearity was not statistically significant so we kept the linear term for simplicity.
Table 3 shows the association between T4 and chronic minor psychiatric morbidity. The odds ratio associated with a 1 standard deviation increase in T4 was higher at 1·21 (95% CI 1·02–1·43; P= 0·03) but was markedly attenuated to 1·11 (95% CI 0·92–1·34; P= 0·27) after adjustment for alcohol, socioeconomic position and smoking behaviour. The inclusion of smoking status made the largest impact on the initial association. It is possible that these variables act as intermediaries rather than confounders as chronic depression may itself increase alcohol consumption, smoking behaviour and result in downward social mobility hence a lower socioeconomic status. If this is true, then the fully adjusted model under-estimates the true association between T4 and minor psychiatric morbidity. Socioeconomic status and smoking were both positively associated whilst age was inversely associated with minor psychiatric morbidity.
We found no real association between T4 and risk of recovered minor psychiatric morbidity. The age adjusted odds ratio was 1·02 (95% CI 0·78, 1·33, P-value 0·87) and this was slightly increased in the fully adjusted model (odds ratio 1·07, 95% CI 0·82, 1·42, P-value 0·65).
There was no evidence against the null hypothesis when FTI was substituted in the analysis and the results did not change qualitatively when the Likert scoring method was used. An analysis based on quartiles of T4 was consistent with a linear effect. Finally, the inclusion of anxiety in the model made minimal difference to the odds ratios; the age-adjusted odds ratio were 1·06 (0·91–1·24, P= 0·46), 1·21 (0·99–1·48, P= 0·06) and 1·01 (0·77–1·32, P= 0·93) for incident, chronic and recovered psychiatric morbidity after adjustment for the STAI.
Our search strategy identified 116 papers. After exclusions, we were left with 13 papers (see Fig. 1). There was one prospective study, six cross-sectional studies and six case–control studies. We excluded the case–control studies, as they had no data on the association of thyroid function and depression in the normal population range. This left us with eight studies (including our own) for potential inclusion into the meta-analysis (see Table 4). In one study, we were unable to contact the lead author and in another case the author would not provide the relevant data, and we did not feel we could use the published material. This left us with nine measures of association from six studies.
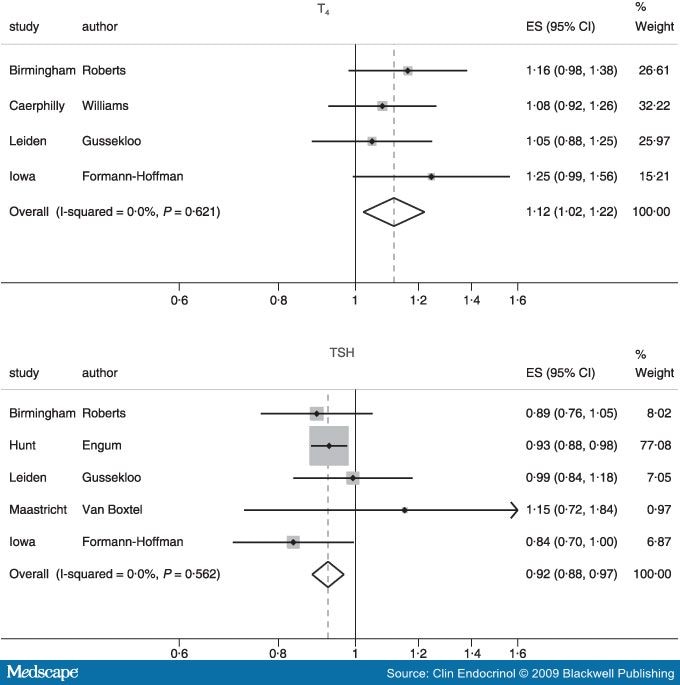
Initially we ran models using whichever measure of thyroid function was available (three authors used T4 and TSH as measures of thyroid function[9,20,21] and two used only TSH[5,22]) divided into quintiles. We then repeated this analysis using a continuous (Z-scored) term. As there was no evidence of nonlinearity we have only presented the results based on the continuous models (Fig. 2). The pooled odds ratios for an increase of one standard deviation change in thyroid function (Z-score) were: 1·12 (95% CI 1·02–1·22; P= 0·01) for T4 and 0·92 (95% CI 0·88–0·97; P= 0·0007) for TSH. For the TSH analysis, the HUNT study[5] provided the majority of the information given its large sample size. There was no evidence of heterogeneity; the I-squared and P-values for T4 and TSH, respectively, are (0·0%, 0·621) and (0·0%, 0·562) To test for small study effects we performed Begg[23] and Egger[24] tests; the latter gave a P-value of 0·25 for T4 and 0·81 for TSH providing no evidence to support the notion of publication bias.

|
Figure 2.
Forest-plots for studies reporting associations between T4 and/or TSH with depression or minor psychiatric morbidity. |
Figure 2.
The study by Forman-Hoffman measured a depression 'syndrome' on the basis of a subjective report of feeling 'depressed, sad or blue' and at least three from eight depressive symptoms.[21] As this is not a validated measure, we repeated our analyses excluding this study. The repeat results were almost the same; 1·10 (95% CI 1·00–1·21, P= 0·06) for T4 and 0·93 (95% CI 0·89–0·98; P= 0·003) for TSH. We also examined for any gender differences in the strength of the association as previous research has suggested that this may be the case.[21] We failed to find any evidence of a gender difference and there was no evidence of heterogeneity (males vs. females: T4 1·10 vs. 1·15, P= 0·67; TSH 0·87 vs. 0·93, P= 0·37).
Discussion
CaPS is the first prospective cohort to examine a possible link between thyroid function with incident, chronic and recovered minor psychiatric morbidity amongst middle-aged men. Our findings showed a weak positive effect of total T4 on incident and chronic psychiatric morbidity, though the former was consistent with chance. This is in the opposite direction from that which is classically taught, i.e. low thyroid function may cause depression. We found no real association between thyroid function and recovery from psychiatric morbidity. However, we would have been underpowered to detect small effects. Our systematic review and meta-analysis further confirms these results for both T4 (positively correlated with depressed mood) and TSH (negatively correlated with depressed mood) thereby enhancing the generalizability of our findings for populations in developed countries.
Strengths and Weaknesses
The main strength of our data is that it comes from a prospective cohort study with a reasonably large sample and long follow-up. We have the unique benefit of having repeat measures of mood and can therefore differentiate minor psychiatric morbidity of new onset, that which is chronic, and recovery from such morbidity. We also have the benefit of being able to adjust for a range of potential confounders, though in the case of chronic minor psychiatric morbidity it is possible that our models adjusted for smoking and alcohol consumption were over-adjusted or 'biased' if thyroid function effects covariates such as alcohol and smoking behaviour either directly or through its association with chronic depression, which in turn effects these life style behaviours. As Hernan and colleagues have shown using directed acyclic graphs, adjusting for a variable that is secondary to the outcome and associated with the exposure will result in misleading estimates of association.[25] Interestingly, adjustment for smoking did not alter the associations in the full model for incident cases as in this case smoking status was more likely to be measured before the onset of morbidity and hence not altered by it.
Because TSH assays were not widely available when this study began, we could only classify thyroid status on the basis of T4 measures. We have therefore had to examine the effects of 'low-normal thyroid function' rather than the more widely accepted notion of 'subclinical hypothyroidism.' The latter is defined as elevated serum TSH in the presence of normal serum T4. It has a prevalence of between 3 and 8%[26] and is known to progress to overt hypothyroidism at a rate of 2·6% per year.[27] However, our findings are consistent with more contemporary studies that do have TSH measured and the associations we see in the meta-analysis are very similar for both T4 and TSH.
Our study was carried out in white males and the results may not be general sable to women or other ethnic groups. However, the consistency of our study with other studies from the meta-analysis adds weight to the view that the association we observed is likely to apply across age groups and we failed to find any difference in our associations from the meta-analysis when comparing men and women.
It is possible that the observed association in CaPS is artefactually increased if our cases include both depression and anxiety and if elevated thyroid function is associated with anxiety. However, there was hardly any alteration in the odds ratios when the STAI was entered into the regression model and our results are consistent with those studies using measures of depression that are less likely to be contaminated by anxiety.
Interestingly, a large cross-sectional study[5] from Norway noted that subjects with both known hyper and hypothyroidism (including those already on thyroxine) were more likely to be depressed. It is possible that some individuals are more dependent on thyroid hormones for their psychological well-being or that diagnosis and/or treatment may worsen psychological well-being.
Our failure to show an inverse association between thyroid function and depression does not necessarily exclude the possibility that thyroid replacement therapy might be beneficial for patients who are hypothyroid or with a normal T4 (and high TSH) in terms of improving mood symptoms and preventing progression to overt hypothyroidism.[28] Reported trials of euthyroid depressed patients having their antidepressants augmented with T3 do not reach a consensus.[29] The association between thyroid function and mood may well differ under normal physiological condition when compared with a deficit disorder. However, it is important to balance any potential benefits with the known adverse effects of thyroxine, such as atrial fibrillation, exacerbation of angina and osteopaenia.
All the epidemiological studies up to now have measured thyroid function on a single occasion and this may not reflect true biological variations across the life course. However, total T4 has a low index of variability for an individual at least over a 12-month period.[30] Functional polymorphisms may provide a better measure of long-term exposure. For example, conversion of T4 to the active 3,5,3'-triiodothyronine (T3) is achieved through enzyme activity both extra- and intracellularly (by T4 5'-deiodinase types I and II, with inactivation performed by a further subtype, type III).[29] Polymorphisms of the single nucleotide and neutral type have already been found in deiodinase genes.[31] Some of these are known to exert an influence on T4 and TSH levels although as yet it is unknown whether the polymorphisms are directly associated with depressed mood. The combination of differential expression of deiodinase subtypes across body tissues and the existence of polymorphisms may infer differential levels of intracellular T3 in different organs.
Similarly, peripheral levels of T4 in blood may not reflect central levels in the brain. Transthyretin is a binding protein crucial to transport and distribution of in the central nervous system (CNS). A study of transthyretin in the cerebrospinal fluid of depressed patients found significantly lower levels than in controls.[32] This lends weight to the view that 'CNS hypothyroidism' could coexist with peripheral (and therefore measured) euthyroidism. However, such testing is not feasible for large population-based studies.
In conclusion, we find no evidence either in the CaPS or other studies that low thyroid function is associated with depression over the life course. In contrast, we provide evidence supported by our meta-analysis that high normal thyroid function (lower TSH and higher T4) is associated with depression. Future research should examine whether deiodinase polymorphisms are related to mood in both 'healthy' subjects and in those treated for hypothyroidism. Currently there is no evidence to treat depressed patients who are euthyroid with thyroxine
No comments:
Post a Comment